Homeostasis of sebaceous gland is androgen dependent so that secretion, differentiation and proliferation of sebocytes is closely interrelated with active androgen molecules such as dehydroepiandrosterone (DHEA), androstandione and testosterone in the skin, which may be considered in homonal acne treatment[1] [2]. In addition, testosterone, major circulating androgen, is converted intracellularly by 5-alpha reductase to dihydrotestosterone (5α-DHT), major tissue androgen [3]. Androgens exerts their effects on sebaceous glands through binding to androgen nuclear receptors [4]. Therefore, the term hormonal acne appears as intuitive as inflammatory acne as acne vulgaris is a disease of pilosebaceous unit which is essentially an endocrine organ.
Interestingly, keratinocytes demonstrate a distinguished role in androgens metabolism in the skin compare to that of sebocytes. Seemingly, catabolism of tissue-active androgens is more efficiently performed in keratinocytes in contrast to sebocytes which are capable of synthesizing androgen receptor binding and tissue-active compounds from the adrenal androgen DHEA, circulating in serum [5]. Schematic model of interaction between androgens and other molecules in differentiation, proliferation and lipogenesis of sebocytes [6].
The human skin, especially the sebaceous gland is a sterioidogenic organ similar to the gonads and adrenal cortex, processing all the enzymes necessary for steroid sex-hormone synthesis and metabolism [7]. This finding is evidenced by detecting sex-hormone gene expression in sebocytes. Immortalization of human sebocytes with SV40 antigen has allowed intensive investigation and advancement in our understanding of sebaceous gland function and homeostasis [5]. Immortalized sebocytes express stereodogenic proteins, cofactors and transcription factors as evidenced by immunohistochemistry and western blot [8]. De novo synthesis of androgens from cholesterol has been suggested by the same study.
Further evidence for androgenesis of sebocytes coming from isotretinoin studies that pertain serum DHT levels to sebocyte activity. Isotretinoin-produced depressions in the serum levels of DHT, believed to be the result, rather than the cause, of a reduction in the size of the sebaceous glands [9]. This evidence explains the amount of tissue-derived androgens that sebaceous glands normally contribute to the circulating pool.
Moreover, synthesis of androgens by sebaceous glands can explain normal serum and urine level of these hormones in acne subjects [10]. Dihydrotestosterone, the most potent androgen, is produced in sebaceous glands by 5-alpha-reduction of testosterone and mediates its effect by binding to its nuclear receptors. Sebaceous glands have receptors for many hormones and substances including nuclear receptors for androgens. Activation of these receptors is associated with differentiation, proliferation and lipid synthesis of sebocytes [11].
The activity of 5-alpha reductase varies according to its location. In sebaceous glands of facial and scalp regions this enzyme demonstrates more activity [12]. This may explain the difference seen in severity of response seen with DHT on facial and non-facial sebocytes. Comparably, more exaggerated response has been experienced with DHT on facial sebocytes and a more modest impact on other regions of the body [13].
Effect of DHT on sebaceous lipogenesis either could not be seen or has been shown to be modest in vitro without presence of an additional cofactor [14]. A peroxisome proliferator-activated receptor (PPAR) ligand such as linoleic acid has been postulated as a cofactor that can promote DHT-mediated lipogenesis. Known for their role in lipid biosynthesis, PPARs are nuclear receptors for hormones with alpha (mostly expressed in keratinocytes), gamma and delta (mostly expressed in sebocytes) subtypes [6].
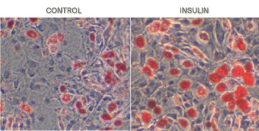
Conversely, LY191704, a 5-alpha-reductase inhibitor has been shown to prevent influence of DHT on sebocytes through manipulation of PPAR [15]. Finally, DHT exerts its effect on sebocyte differentiation through PPAR-gamma [16]. Experimentally, rosiglitazone has been shown for having a synergistic effect with DHT in sebaceous cell differentiation [17]. Sebocytes differentiation after DHT treatment has been compared to that of thiozolidinedione. Synergistic effect of DHT and rosiglitazone is seen in very left column [17].
Another aspect of involvement of androgens in pathogenesis of hormonal acne vulgaris may be explained by eliciting an inflammatory response. Up-regulation of interleukin-6 (IL-6) and TNF-alpha in immunohistochemistry, and increase in RNA amplification for IL-6 and tumor necrosis factor (TNF-alpha) were observed after addition of DHT compared with the control [18].
Other than androgens such as dehydroepiandrosteone (DHEAS) and dihydrotestosterone (DHT), other hormones may contribute to hormonal acne formation and severity . Insulin-like growth factor (IGF-1) may influence hormonal acne in adult men and women with stronger effect on acne in women through a reciprocatory role with androgens [19]. IGF-1 stimulates 5-alpha-reductase, adrenal and gonadal androgen synthesis, androgen receptor signal transduction, sebocyte proliferation and lipogenesis [20]. Treatment with metformin, diets low in milk protein and glycemic index reduce IGF-1 signaling with improvement in acne lesions [21].
A study which compared homone-mediated sebocyte differentiation with that of adipocytes concluded that while there exists similarities, the distinctions are dramatic. During sebocytes or adipocytes differentiation various lipids, mainly triglycerides,, TGs, accumulates within droplets which eventually burst liberating lipids and cell debris, a process facilitated by insulin and manipulated by androgens. While androgens boom sebocyte differentiation and amplify the skin lipogenesis, they dwindle adipocyte maturation. In contrast, vitamin D3, 1,25(OH)D3, lowers insulin-intervened lipid production within sebocytes, their differentiation and holocrine secretion. On the other hand, with low extracellular calcium and vitamin D keratinocytes do not differentiate and their apoptosis is languished while their proliferation intensifies leading to aggravation of inflammatory acne.
Use of inhibitors of dihydrotestosterone (DHT) has been shown effective in prevention of sebaceous gland growth and elucidating apoptosis in patients with acne vulgaris. Studies by Hoffman et al, have provided evidence for selective induction of apoptosis in the sebaceous gland of hamster flank by topical application of liposome 5-alpha-reductase inhibitor [22]. Growth of sebaceous gland in hamster flank is stimulated by androgens [23]. Topical application of unsaturated fatty acids such as linolenic acid to the testosterone-treated flank organ suppressed this testosterone effect and may be found effective in treatment of hormonal acne [24]. This effect is mediated through inhibition of dihydrotestostrone [24].
Increase in plasma androgen level induced by steroids cause an increase in cholesterol and free fatty acids in skin surface in conjunction with sebocyte hypertrophy [25]. Some essential fatty acids such as gamma linolenic acid are potent 5-alpha-reductase inhibitors and hypothetically by inhibition of androgen receptors can be effective in treatment of acne [23]. In adult skin androgen action is dependent to conversion of testosterone to five-alpha-dihydrotestosterone [26]. This conversion takes place under enzymatic act of five-alpha-reductase. Hypersensitivity of androgen receptors in pilosebaceous duct and the resulting increase in sebum production appears to be associated with a significant increase in free fatty acids rather than other surface skin lipids and should be considered in hormonal acne treatment.
Use of anti-androgen hormonal treatment such as combination oral contraceptives/cyproterone acetate have been associated with alleviation of acne with suppression of androgen metabolizing skin cells, follicular keratinocytes and sebocytes, causing sebostasis [27]. However, their use is limited to patients who have exhausted other treatment options such as isotretinoin or have shown evidence of hormonal imbalance [28].
A persistent dialogue between seobocytes and androgens influences sebaceous glands pathophysiology. Sebocytes differentiation, proliferation and secretion is directly affected by change in skin androgen levels and implicates in hormonal acne treatment.
The skin is equipped with enzymes to synthesize androgens such as DHT and dihydroandrostandione from endocrine DHEA. As a result local epidermal androgen excess may be present concurrent with normal serum testosterone/DHEA level. Another aspect of this process is mediation of PPAR-gamma in interaction of androgens with sebocytes. Agonists and antagonists of PPAR-gamma work in conjunction with androgens to finalize impact of androgens on androgen nuclear receptors.
Androgens proinflammatory effect and their influence on lipogenesis seem to be two confluent yet distinct streams, which ultimately lead to acne exacerbation or comedone formation. Moreover, Increase in lipogenesis is associated with not only a quantitative increase in epidermal fatty acids but also an alteration of skin surface lipids, which exaggerates an inflammatory response at sebocyte level. On the other hand, androgens may illicit an up-regulation in cytokines such as IL-6 and TNF-alpha.
Admittedly, androgens represent a more significant role in pathogenesis of acne vulgaris, yet, IGF-1 studies have brought about great advances in understanding of sebaceous gland disorder. IGF-1 influences 5-alpha-reducatase and manipulates level of DHT in tissues and further affects sebocytes homeostasis. More importantly, IGF-1 and insulin exerts their effects by activating mTORC1 signaling, activation of SREBP and increase in lipogenesis. Another aspect of role of mTORC1-SREBP signaling is influence of this pathway on androgen synthesis at sebocyte level, further aggravating acne. Strikingly-different from what has been governed acne literature for quite long time is mTORC1 studies. This revolutionary idea brings insight into the way we handle recommendation and counseling in hormonal acne treatment and regarding their diet.
Importance of androgen mechanisms at sebocytes level is mainly in that hormonal acne treatment could be modified accordingly. Pharmacological antagonist of 5-alpha-reductase and anti androgens characterize major available treatment limited to use for severe forms of acne vulgaris such as cystic acne as well as those with evidence of androgen excess. However, gamma linolenic acid and DHA/EPA have been subject of multitude of studies which suggest their therapeutic value in DHT manipulation and their substantial place in maintenance treatment of acne vulgaris.
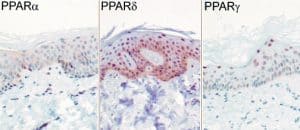
PPAR subtypes, nuclear hormone receptors, expressed in keratinocytes and sebocytes
Expression of PPAR (peroxisome proliferator receptor) subtypes in human sebocytes. [29]. PPAR-alpha mediates early lipogenic steps common to the function of both keratinocytes and sebocytes, PPAR-gamma plays a unique role in stimulating sebocyte lipogenesis and PPAR-delta activation may contribute to lipid biosynthesis in both sebocytes and keratinocytes [6].
1. Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113-C118.
2. Diamond P, Cusan L, Gomez JL, Belanger A, Labrie F. Metabolic effect of 12 months percutaneous DHEA replacement therapy in postmenopuasla women. J Endorcrinol. 1996;150:S43-S50.
3. Wilson JD, Walkder JD. The conversion of testosterone to dihydrotestosterone by skin slices of man. J Clin Invest. 1969;48:371-9.
4. Janne OA, Bardin CW. Androgen and antiandrogen receptor binding. Annu Rev Physiol. 1984;46:107-118.
5. Fritsch M. Orfanos C, Zouboulis CC. Sebocytes are the key regulators of androgen homeostais in human skin. J Invest Dermatol. 2001;116:793-800.
6. Deplewski D, Rosenfield RL, Greene ME. Peroxisome proliferator-activated receptors and skin development. Horm Res. 2000;54(5-6):269-74.
7. Chen W, Zouboulis CC, et al. Expression of sex-determining genes in human sebaceous gland and their possible role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2006;20(7):846-52.
8. Thiboutot D, Zhaoyuan Cong, Gary Clawson, et al. Human skin is a steroidogenic tissu, steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes and an immortalized sebocyte
cell line (SEB -1). J Invest Dermatol. 2003;120:904-14.
9. Lookinngbill DP, Demers Lm, Tigelaar RE, Shalita AR.
Effect of isotretinoin on serum levels of precursor and peripherally derived androgens in patients with acne. Arch Dermatol. 1988;124(4):540-3.
10. Forstrom L. The influence of sex hormones on acne. Acta Derm Venereol Suppl (Stockh). 1980;Suppl 89:27-31.
11. Zouboulis. Sebaceous gland receptors. Dermatoendocrinol. 2009;1(2):77-80.
12. Takayasu S, Wakimoto H, Itami S, Sano S. Activity of testosterone 5-alpha-reducates in various tissues of human skin. J Invest Deramtol. 1980;74:187-91.
13. Akamatsu H, Zouboulis CC, Orfanos CE.
Testosterone and 5-alpha-dihydrotestosterone is dependent on the localization of the sebacous glands. J Inves Dermatol. 1992;99(4):509-511.
14. Guy R, Ridden C, Kealey T.
The improved organ maintenance of the human sebocyte gland, modeling in vitro effect the effects of epidermal growth factor, androgens, estrogens, 13-cis retinoic acid, and phenol red. J Inevest Dermatol. 1996;106:454-60.
15. Makrantonaki E, zouboulis CC.
Testosterone metabolism to 5-alpha-dihydrotestosterone and syntheis of sebacious lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. Br J Dermatol.
2007;1156(3)428-32.
16. Rosenfield RL, Deplewski D, Kentsis A, Ciletti N. Mechanisms of androgen induction of sebocyte differentiation. Dermatology. 1998;196(1):43-6.
17. Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev. 2000;21(4):363-92.
18. Lee WJ, Jung HD, Chi SG, Kim BS, et al.
Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. 2010;302(6):429-33.
19. Cappel M, Mauger D, Thiboutot D.
Correlation between serum levels of insulin-like growth factor 1, dehydroandrosterone sulfate and dihydroepiandroestrone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333-8.
20. Melnik BC, Schmitz G.
Role of insulin, insulin-like growth factor-1, hyperglycemic food and mild comsumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833-41.
21. Melnik BC.
Milk consumption, aggravating actor of acne and promoter of chronic diseases of western societies. J Dtsch Dermatol Ges. 2009;7(4):364-70.
22. Li L, Tang L, Baranov E, Yang M, Amoh Y, Katsuoka K, Hoffman RM.
Selective induction of apoptosis in the hamster flank sebaceous gland organ by a topical liposome 5-alpha-reductase inhibitor. J Dermatol. 2010;37(2):156-62
23. Liao S, Lin J, Dang MT, Zhang H, Hippakka RA. Growth suppression of hamster flank organs by topical applicatioin of catechins, alizarin, curcumin, and myristoleic acid. Arch Dermatol Res. 2001;293(4):200-5.
24. Liang T, Liao S.
Growth suppression of hamster flank organs by topical application of gamma-linolenic and other fatty acid inhibitors of 5 alpha reductase. J Invest Dermatol. 1997;109(2):152-7.
25. Scott MJ 3rd, Scott AM. Effects of anabolic-androgenic steroids on the pilosebaceous unit. Cutis. 1992;50(2):113-6.
26. Liao S. Androgen action, molecular mechanism and medical application. J Formos Med Assoc. 1994;93(9):741-51.
27. Rabe T, Zouboulis CC. Hormonal antiandrogens in acne treatment. J Dtsch Dermatol Ges. 2010;8 Suppl:S60-S74.
28. Thiboutot D. Acne, hormonal concepts and therapy. Clin Dermatol. 2004;22(5):419-28.
29. Westergaard M, Henningsen J, Svendsen ML, Johansen C, Jensen UB, Schroder HD, Kristiansen K, et al.
Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J Invest Dermatol. 2001;116:702-12.